This story was produced by FairWarning, a nonprofit news organization based in Southern California that focuses on public health, consumer, labor and environmental issues. You can sign up for their newsletter here.
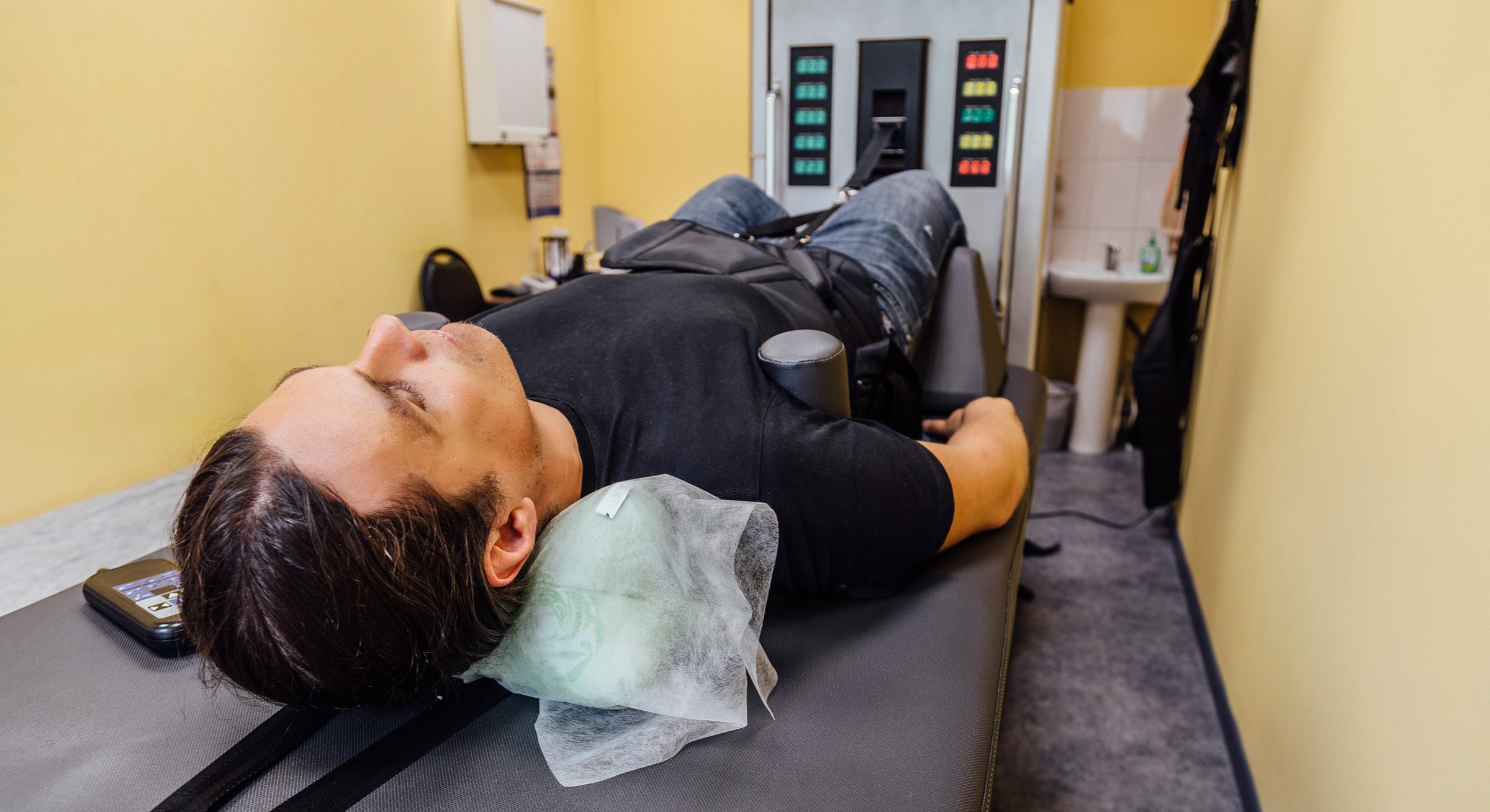
Desperate to relieve their suffering, people with chronic back pain who comb the internet looking for help sometimes stumble upon a device called the DRX9000.
It’s a mechanical table attached to Space Age-looking controls that its manufacturer claims can stretch the disks of the vertebrae, allowing bulges and herniations to be pulled back into place and taking pressure off nerve roots.
One Pennsylvania woman wrote on the DRX9000 Facebook page that she could barely stand long enough to take a shower or wash dishes because of bulging and torn disks.
“I suffer everyday and I’m disabled because of it,” she wrote. “What should I do?”
On Facebook and its website, the company behind the DRX9000, Excite Medical, offers compelling answers. Nearly 9 out of 10 patients who qualify for treatment on the DRX9000 will get relief, the company says. And it claims that researchers affiliated with prestigious institutions, including Stanford, Johns Hopkins and the Mayo Clinic, have done studies that “demonstrated” or “documented” its effectiveness.
The DRX9000 is one of more than a dozen “spinal decompression” devices that for three decades have offered back patients the tantalizing prospect of relief. Excite Medical, which calls the DRX9000 the industry leader, says that 2,400 of its systems are in use in 45 nations and shows it off at trade shows everywhere from Las Vegas to Dusseldorf, Germany, and Dubai, United Arab Emirates. Chiropractors across the United States buy the machines from Excite Medical and the makers of several similar brands and market the treatment, often using the same claims as the manufacturers — sometimes even going beyond them.
But a FairWarning investigation — based on review of lawsuits, scientific studies, government documents, chiropractic websites and interviews with experts — found that the claims of success for spinal decompression stretch the truth, enticing patients to pay thousands of dollars for a treatment that has never been proven in scientifically rigorous studies to live up to its stupendous billing.
Despite a spate of state regulatory actions in the 2000s against Axiom Worldwide, the original manufacturer of the DRX9000, and chiropractors for making unproven claims, they still permeate the internet. And federal and state regulators who can sanction false claims now show little evidence that they are interested in reining them in, the investigation found.
“Some may say that it is too good to be true, but research indicates that 92% [of] patients report overall improvement,” Shasta Spine Specialists in Redding, California, says of the DRX9000 on its website. The clinic, which cited a 1998 study of a different machine that Aetna described in a policy bulletin as “poorly designed” and without a control group, did not respond to a request for comment.
“This non-surgical spinal decompression system … is scientifically Proven By Mayo Clinic, Duke University, Stanford, and Johns Hopkins University School of Medicine!” according to the website for GO Chiropractic in Illinois, which offers treatment with the DRX9000.
Jamie Stephens, one of the chiropractors who runs Go Chiropractic, said in an email, “We have seen nothing but outstanding results from this technology,” and referred further questions to Excite Medical, which he said provided his advertising materials.
Saleem Musallam, president of Excite Medical, said in an interview that the DRX9000 has saved countless people from unnecessary surgery and improved their lives. “I can tell you that you will not find a single person out there to tell you the DRX doesn’t work,” he said.
Musallam acknowledged, however, that more research is needed on spinal decompression in general.
Though other spinal decompression brands were not subject to the same level of scrutiny from regulators, many chiropractors who offer treatment with the devices make similar claims of success, citing studies that have been rejected by insurance companies and Medicare as less than scientifically sound.
For the DRX9000, most of the studies by doctors affiliated with the prestigious universities cited on Excite Medical’s website report promising results such as reduced pain and better functioning. But all eight studies call for more rigorous scientific research, including assigning patients randomly to groups getting treatment or a placebo, to prove the device’s worth. One of the studies’ authors says he has even demanded in a cease-and-desist letter that Excite take his studies off its website because Excite has no rights to his intellectual property. (Musallam declined to comment on the cease-and-desist.)
Insurance companies generally won’t pay the cost of spinal decompression treatment — which Excite Medical says typically runs about $3,500 for a full course of sessions on the DRX9000 — because they say there is no proof it works. Medicare won’t cover it, either.
Aetna, in its policy bulletin, calls spinal decompression “experimental” and “investigational.”
“Currently, there is no adequate scientific evidence that proves [it]… is an effective adjunct to conservative therapy for back pain,” according to the bulletin updated Oct. 1, which reviewed studies going back to 1998. In addition, the devices “have not been adequately studied as alternatives to back surgery.”
The DRX9000 Facebook page includes comments from patients who swear by it.
“I had bulging discs so bad I couldn’t stand up straight or walk,” one South Carolina woman wrote. “Had to use a wheelchair. My chiropractor got me on this and I thank God. After a week I was able to use a walker. After another week I was walking on my own.”
But Stephen Barrett, a retired doctor who founded the website Quackwatch to debunk false medical claims, is skeptical that more rigorous research will support the claims of a 90 percent success rate.
“If this device could actually relieve 9 out of 10 people,” he said, “it would be making headlines everywhere.”
[Read more about how the author got the story.]
‘Worthless’ research used to lure patients
Back pain has long plagued humankind. Over the course of a lifetime, 80 percent of people will experience it, with 15 to 20 percent reporting a back episode in the past year. It’s the second most common reason for seeking medical attention, according to the Cleveland Clinic.
Most back pain resolves on its own within a few months. But chronic cases can upend a person’s ability to work and enjoy life. It’s the most common reason for disability in people under the age of 45.
The spinal decompression industry came to life in the 1990s when a former Canadian government health official named Allan Dyer started marketing a device called the VAX-D that he claimed could lower pressure in disks.
Imitators soon entered the market, and some of the people behind the new devices split off and formed their own companies. The result was more than a dozen, with high-tech-sounding names like Accu-SPINA, Antalgic-Trak and Triton DTS. Before the DRX9000, there were the DRX2000, DRX3000 and DRX5000.
By the late 2000s, Axiom Worldwide’s DRX9000 appears to have pulled ahead of the pack, industry insiders say, thanks perhaps to an aggressive marketing plan. Chiropractors who paid as much as $125,000 for the device also got a package of suggested promotional materials, including the claim the DRX9000 was used in a scientific study that showed an 86 percent success rate. Many of the chiropractors took out newspaper ads that included the claims.
In later lawsuits, chiropractors complained that they were duped by Axiom. One, James Spiering in Texas, described being flown, plane fare and hotel paid, to Axiom headquarters in Florida, where he was told he would recover his investment in four months and clear $1.7 million in five years.
Spiering said he was shown videos full of “fraudulent” claims. The parties settled out of court in 2010 for an undisclosed amount.
Regulators across the U.S. also had started to take notice of the DRX9000’s claims of extraordinary success. Over the course of three years or so, the Oregon attorney general, the Florida attorney general and a group of 11 California district attorneys all filed suits against Axiom or a former chiropractor who created some of its marketing. The suits ended in penalties — $1.125 million in the California case — and Axiom agreed to only make claims based on reliable scientific evidence, according to news stories and settlement documents.
One of the claims the regulators targeted was from a 2003 study by Dr. Thomas Gionis that found 86 percent of patients treated with an unnamed spinal decompression device experienced an “immediate resolution of symptoms.”
The Florida attorney general, in its 2009 lawsuit against Axiom Worldwide accusing the company of deceptive and unfair trade practices, pointed out that the Gionis study lacked a control group and combined spinal decompression with other types of treatment. (Axiom was using the study in its promotions even though the study did not specify what type of spinal decompression table it tested.) Six years later, without admitting any violations of the law, Axiom agreed to a permanent injunction promising only to make any claims based on “competent and reliable scientific evidence” and to reimburse the attorney general $19,000 for its costs.
Not only was the Gionis study far from scientifically rigorous — the company also withheld information about the doctor himself that tended to cast doubt on the findings, according to two lawsuits against Axiom.
Before he did the study, Gionis had been sentenced to five years in prison for hiring two men to assault his estranged wife — the daughter of film legend John Wayne — and her boyfriend. The men sent by Gionis bound and roughed up the two victims, and slashed the boyfriend’s Achilles tendon, according to the charges. His medical license was later put on probation as a result of the conviction.
Gionis, who maintained his innocence in the assault on his wife, did not respond to a request for an interview.
Musallam, who worked at Axiom before starting Excite Medical, eventually winning the intellectual property rights to the DRX9000 through protracted litigation, called the Gionis study “worthless” and said he didn’t use it.
“We try to stick to hard facts and things that are credible,” he said.
Yet it’s easy to find scores of chiropractic office websites that do, including those that offer treatment with the DRX9000 and also other popular brands of spinal decompression machines. Some reproduce the entire Gionis report, while others refer to it by name or cite the 86 percent “success rate.”
“Decompression 86% Effective,” reads the headline over the Gionis study on the website of Natural Spine Care in Dublin, California, which offers treatment on a different device called ABS.
Jim Yang, one of the chiropractors there, said that “the people we buy it from provide that information,” and that he would have expected them to do their due diligence about the study’s validity (ABS is no longer in business). Yang added that “people do very well” with the treatment, and he cited one patient who’d been told he would never ski, golf or run after back surgery but is now doing all three.
As the Gionis study came under fire from regulators, Axiom realized it needed new data and formed a medical advisory board to do additional studies, Musallam said.
But the research, in many cases funded by Axiom, included big caveats: Because it lacked scientific rigor, including double-blinding in which neither doctors nor patients know who was randomly assigned real treatment versus placebos, no definite conclusions could be drawn.
The studies have another shortcoming, said Richard Deyo, professor emeritus at Oregon Health and Science University who has studied low back pain and inappropriate uses of medical technology, and who has reviewed the studies.
Eight in 10 people with back pain get better on their own, he said. So how to tell if those treated with spinal decompression would have improved without it?
Complaints of injuries
Spinal decompression is often advertised as a safe alternative to surgery. But several lawsuits and FDA documents show that patients have alleged serious injuries from the devices.
In July, Charlene Vaught of Florida sued Massage and Spinal Therapy of Winter Haven and owner Angie Reynolds, alleging that she experienced severe neck pain, atrophy in both hands and difficulty with motor skills after a treatment on a DRX9000 by an office assistant. Vaught says she now needs a home health aide.
The company has denied Vaught’s allegations. It did not respond to a request for comment.
On the DRX9000 Facebook page, more than a year before the lawsuit was filed, Reynolds claimed that in her 15 years of treating patients she had chalked up a 96 percent “success” rate, though she didn’t describe what that meant.
“I personally had three failed spine surgeries,” she wrote, “and the DRX 9000 is what finally cured my back pain. It is safe, it is effective, and it definitely is life-changing for most all of my patients.”
That case is still being litigated, but others have resulted in damages.
In 2010, for instance, a federal judge awarded a New Jersey woman, Marlene Newman, $380,000 from Axiom Worldwide in a default judgement after she suffered a torn rotator cuff during a DRX9000 treatment and had to have three surgeries.
The FDA has received about two dozen complaints about malfunctions in spinal decompression devices manufactured by various companies, some of which resulted in injuries. In 2010, a patient reported pain with every step after 20 treatments on the DRX9000. The patient described it as a “modern version” of a medieval “torture device.”
Many of the FDA complaints are about the Triton DTS machine. One alleged that in 2018, a rope attached to a patient’s harness pulled so hard that the patient had to be taken to an emergency room. A patient in 2015 described losing feeling in the legs and wrote, “It felt like my lower body was separated in two pieces.” The patient continued to have complications a year-and-a-half later, according to the complaint. DJO, the manufacturer in Vista, California, did not respond to a request for comment.
The FDA did not immediately respond to a request for documents showing what actions, if any, it took in these cases, but said that in general it requires device manufacturers to investigate “adverse events” and that the complaints are one tool the agency uses in deciding whether to take further action.
Taken together, the lawsuits and reports do not document widespread injuries from the devices, but they do undermine the claim, made by many practitioners, that spinal decompression is free of risk.
Musallam, president of Excite Medical, said he was unaware of any reports of injuries. “If someone is following its intended use and they’re paying attention to the contraindications, I don’t see how it’s possible they can possibly be hurt by the machine,” he said.
A lack of oversight
The federal agencies with the authority to sanction unproven claims, the Federal Trade Commission and the Food and Drug Administration, appear to have done little to rein in either manufacturers or practitioners.
Barrett, the Quackwatch founder, says that over the years he has contacted the FDA several times about advertising for spinal decompression, as well as other medical devices. He’s never heard back.
“They don’t seem to want to regulate devices,” he said. “I can’t think of any logical reason why not. I mean, spinal decompression is a big industry.”
In response to FairWarning’s questions, an FDA spokeswoman released a statement saying, “When FDA receives information such as a complaint or allegation about a device, the agency will follow up as appropriate.”
In two cases, in 2011 and 2015, the FDA forced spinal decompression manufacturers to recall promotional literature that recommended uses or promised results beyond what the agency approved. But a search of FDA “warning letters” did not turn up the names of any of the major spinal decompression brands. It’s possible that the FDA took other types of action, but the agency did not immediately respond to a request under the Freedom of Information Act for records that would show that.
For drugs that it approves, the FDA requires three randomized clinical trials. But devices that are merely “cleared” by the FDA — meaning that the FDA finds they’re essentially the same as others already on the market — don’t need to have any trials at all, said Deyo, the Oregon professor.
“And so most of these things actually never undergo any sort of rigorous research,” Deyo said.
Today, some chiropractors still advertise that spinal decompression devices are “approved” rather than “cleared” by the FDA even though regulators a decade ago required manufacturers and practitioners to stop using that terminology.
The FTC has not issued any warning letters to manufacturers of spinal decompression devices over the past two decades or taken other public action. The FTC declined to comment.
At the state level, there’s been little action since the 2000s, when several states cracked down. At the time, state chiropractic boards also disciplined individual chiropractors for flimsy claims in spinal decompression advertising, including ones in Florida, California, North Carolina, Kentucky and Wisconsin.
But the state authorities seem to have moved on. Eight state chiropractic boards contacted for this investigation, including several that had sanctioned chiropractors a decade or more ago, could not point to any cases in recent years.
Deyo notes that one of the studies on Excite’s own website looked back at seven earlier studies of spinal decompression that did include randomized control groups. Six of the seven found no difference between those who got spinal decompression and those who did not. The seventh reported lower pain scores but the patients were no less disabled.
“So far,” Deyo said, “the best evidence suggests there’s not much of an advantage of these things.”